- Research
- Open access
- Published:
Establishment of a co-culture system usingEscherichia coli一个dPichia pastoris(Komagataella phaffii) for valuable alkaloid production
188bet亚洲体育平台volume20, Article number:200(2021)
Abstract
Background
Plants produce a variety of specialized metabolites, many of which are used in pharmaceutical industries as raw materials. However, certain metabolites may be produced at markedly low concentrations in plants. This problem has been overcome through metabolic engineering in recent years, and the production of valuable plant compounds using microorganisms such asEscherichia colior yeast cells has been realized. However, the development of complicated pathways in a single cell remains challenging. Additionally, microbial cells may experience toxicity from the bioactive compounds produced or negative feedback effects exerted on their biosynthetic enzymes. Thus, co-culture systems, such as those ofE. coli–E. coli一个dE. coli-Saccharomyces cerevisiae, have been developed, and increased production of certain compounds has been achieved. Recently, a co-culture system ofPichia pastoris(Komagataella phaffii) has gained considerable attention due to its potential utility in increased production of valuable compounds. However, its co-culture with other organisms such asE. coli, which produce important intermediates at high concentrations, has not been reported.
Results
Here, we present a novel co-culture platform forE. coli一个dP. pastoris. UpstreamE. colicells produced reticuline from a simple carbon source, and the downstreamP. pastoriscells produced stylopine from reticuline. We investigated the effect of four media commonly used for growth and production ofP. pastoris, and found that buffered methanol-complex medium (BMMY) was suitable forP. pastoriscells. Reticuline-producingE. coli细胞也表现出更好的增长和reticuline production in BMMY medium than that in LB medium. De novo production of the final product, stylopine from a simple carbon source, glycerol, was successful upon co-culture of both strains in BMMY medium. Further analysis of the initial inoculation ratio showed that a higher ratio ofE. colicells compared toP. pastoriscells led to higher production of stylopine.
Conclusions
This is the first report of co-culture system established with engineeredE. coli一个dP. pastorisfor the de novo production of valuable compounds. The co-culture system established herein would be useful for increased production of heterologous biosynthesis of complex specialized plant metabolites.
Background
Specialized metabolites produced by plants, also known as secondary metabolites, exhibit diverse chemical structures and biological activities. Several metabolites have been used as drugs, such as morphine for the development of analgesics, artemisinin used as an anti-malarial drug, and vinblastine used as an anti-cancer drug [1]. Though more stable industrial production and supply of such useful compounds is necessary, many of the metabolites are unsuitable for chemical synthesis owing to their complex chemical structures and laborious extraction procedures from plants. Owing to the decrease in plant resources and their low concentrations in plant cells, the stable supply of some such compounds may be challenging in the future. To solve these problems, biosynthetic enzymes have been investigated, with their corresponding genes identified and isolated. In recent years, metabolic engineering, a process in which genes for biosynthetic enzymes are introduced into microorganisms such asEscherichia coliorSaccharomyces cerevisiae, leading to successful production of valuable compounds has been reported [2,3]. Few examples are as follows: the production of thebaine, an important opiate, byE. coli[4] andS. cerevisiae[5];the production of tropane alkaloids, that act as neurotransmitter inhibitors, byS. cerevisiae[6];一个d the production of resveratrol, a stilbene with potential health-promoting benefits, byE. coli一个dS. cerevisiae[7].
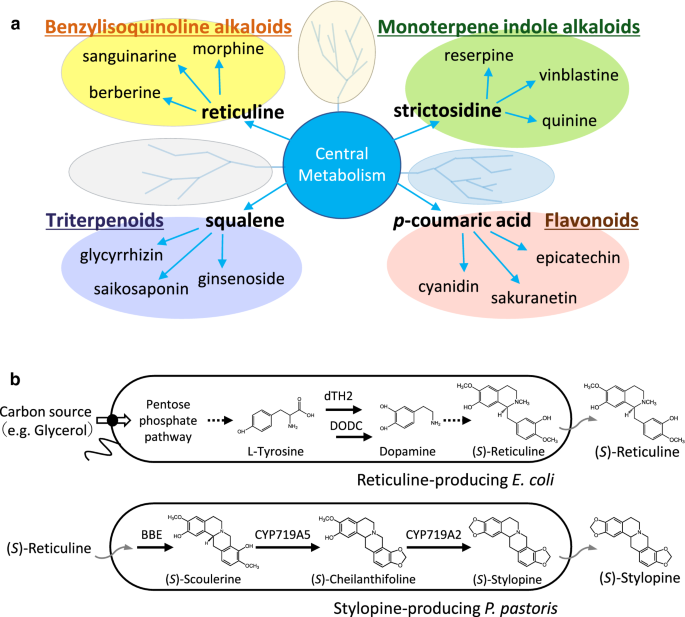
Contrary to successful reports, there are a few compounds that cannot be biosynthesized at high concentrations in microorganisms, probably due to the cytotoxicity of substrates added to the medium or end-products, or the exertion of negative feedback on the biosynthetic enzymes. Additionally, the construction of the entire biosynthetic pathway, including the selection of the most suitable host cell, introduction of multiple biosynthetic genes, and examination of enzyme expression conditions, in a single cell, involves considerable efforts and is time-consuming. To circumvent these problems, we focused on the fact that several specialized metabolites are derived from common intermediates. Various benzylisoquinoline alkaloids (BIAs) are derived from reticuline, and different monoterpeneindole alkaloids originate from strictosidine; furthermore,p香豆交流id helps in the generation of phenylpropanoids including flavonoids, and triterpenoids are derived from squalene [8] (Fig.1a). Therefore, establishment of a co-culture system of microorganisms, each possessing complementary or split pathways, may be a useful strategy for the efficient production of valuable compounds [9,10,11]. In recent years, several reports have demonstrated the co-culture system to be a powerful tool for large-scale production of various compounds. Notable examples include biosynthesis of sakuranetin [12] or anthocyanins [13] using co-culture ofE. coli–E. coli(strains engineered with different genes), and biosynthesis of resveratrol [7], or magnoflorine [14] using a co-culture ofE. coli一个dS. cerevisiae. Recently, a co-culture system withCorynebacterium glutamicum一个dE. colihas also been reported for the production of lysine-derived metabolites, cadaverine, andl-pipecolic acid [15].
Metabolites biosynthesized in plants (a) and cells used for de novo stylopine production (b).aVarious specialized metabolites are produced from common intermediates like reticuline, strictosidine,p香豆交流id, squalene, and others. These intermediates are derived from central metabolism.bReticuline-producingE. colicells (AN2014 strain) produce (S)-reticuline using simple carbon sources such as glucose or glycerol, via three engineered pathways, namely (1) anl-tyrosine-overproducing pathway, (2) a dopamine and 3,4-dihydroxyphenylacetaldehyde (3,4-DHPAA) production pathway froml-tyrosine, and (3) a reticuline-producing pathway from dopamine. Stylopine-producingP. pastoris(B52 strain) produce (S)-stylopine from (S)-reticuline via three steps catalyzed by berberine bridge enzyme (BBE), cheilanthifoline synthase (CYP719A5), and stylopine synthase (CYP719A2)
Novel co-culture systems could be employed to produce compounds hitherto not investigated or could not be generated through the already known combinatorial systems.Pichia pastoris(Komagataella phaffii) is a methylotrophic yeast that has been used for industrial scale production of recombinant proteins.P. pastorishas also been used for the production of valuable compounds in recent years owing to an increased expression of biosynthetic enzymes [16,17,18]. Compounds successfully produced usingP. pastorisinclude lovastatin [19], dammarenediol-II [20], nootkatone [21], ambrein [22], stylopine [23], amongst others. The advantage of usingP. pastorisas a host cell is that certain enzymes that are not functional or exhibit low activity in other organisms present with a high conversion rate in this cell. For example, cytochrome P450 enzyme CYP719A5 derived fromEschscholzia californica, which catalyzes the conversion of scoulerine to cheilanthifoline, showed a higher conversion rate inP. pastoriscells (70%) compared toS. cerevisiaecells (20%) [23]. The construction of the entire biosynthetic pathway, however, is challenging in a singleP. pastoriscell. Therefore, a splitting pathway was proposed and a co-culture ofP. pastoris–P. pastoriswas performed with different cells synthesizing different enzymes [19,23]. However, knowledge of co-culture systems ofP. pastoriswith other organisms is limited; particularly, its co-culture method withE. colihas not been established.E. coliis a standard microorganism used for industrial-scale production of different compounds [24,25], and high production of certain common intermediates for specialized metabolites, such as reticuline [26],p香豆交流id [7], and squalene [27] byE. colicells has been reported. However,E. coliis a prokaryotic cell and lacks subcellular organelles essential for the expression and function of certain enzymes such as cytochrome P450. Therefore, in such cases where further modification of the basic structure of a metabolite through enzymes such as P450 is required, use of eukaryotic cells is more suitable. As described above,P. pastorisshows high protein expression and a higher conversion rate thanS. cerevisiaein a few cases. Therefore, a co-culture system forP. pastoris一个dE. colimay be useful for the increased production of valuable compounds.
In this study, we established a co-culture system forE. coli一个dP. pastoris. Four vectors harboring 14 genes were introduced inE. coli, the upstream strain, to enable production of (S)-reticuline, an important intermediate for various BIAs, using a simple carbon source such as glucose or glycerol [26] (Fig.1b) (Additional file1: Table S1). The downstream strain,P. pastoris, which was established via the introduction of threeE. californicagenes, namely berberine bridge enzyme (BBE), cheilanthifoline synthase (CYP719A5), and stylopine synthase (CYP719A2), into the genome, resulted in the production of (S)-stylopine, a potential anti-inflammatory compound [28], from (S)-reticuline [23] (Fig.1b) (Additional file1: Table S1). Here, we first investigated the effect of several media onP. pastoriscell growth and the biosynthesis of stylopine. We then determined the optimum medium and initial inoculation ratios for co-culture. We report the first establishment ofE. coli-P. pastorisco-culture system, which can be used to produce (S)-stylopine from glycerol. This platform would be helpful for conducting combinatorial biotransformation of a variety of useful components (Fig.1).
Results and discussion
Appropriate medium for stylopine production inP. pastoris
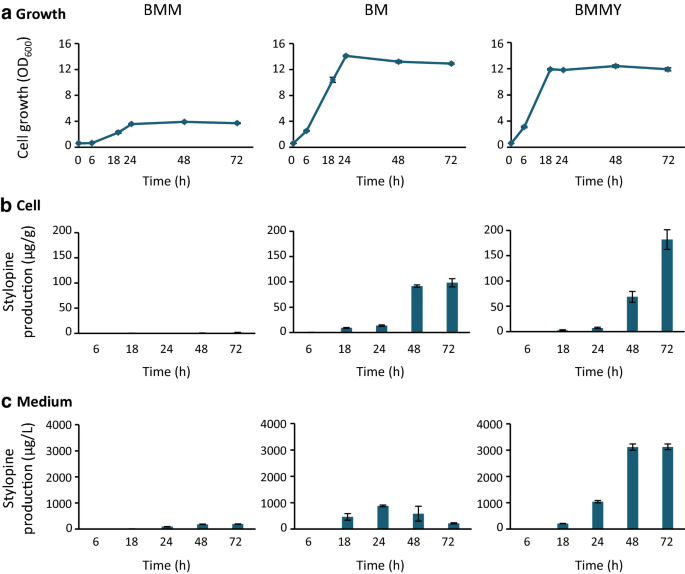
ForP. pastorisculture, few basic media (Table1), such as minimal methanol (MM), buffered minimal methanol (BMM), and buffered methanol-complex medium (BMMY), developed by Invitrogen Co. have been commonly used. However, knowledge about the effects of these media on the production of stylopine is limited. Therefore, we tested these three media and BM medium, the use of has been reported in a previous study [29),确定一个合适的媒介,我们可以ed for cell growth and stylopine production (Table1).Stylopine-producingP. pastoriscells, pre-cultured in YPD medium, were inoculated at OD600= 0.6, in each medium containing reticuline as a substrate, and incubated at 30 °C under shaking conditions (250 rpm). The cell growth and stylopine biosynthesis were monitored. Since stylopine production was not observed in MM medium (Additional file2: Fig. S1), the data in other media are shown (Fig.2).Cells cultured in other media exhibited exponential growth up to 18–24 h, and then entered the stationary phase (Fig.2a). Early induction of the stationary phase and limited cell growth was observed in the BMM medium, compared to other media. It should be noted that stylopine concentrations in cells and medium differed significantly between media (Fig.2b, c). Although stylopine was present in the BMM medium, which is a buffered MM medium (Table1), its production rate was the lowest compared to other media. It could be inferred that stylopine production might be influenced by the pH of the medium. In the BM medium, stylopine was produced at a higher concentration and was predominantly accumulated in cells after 48 h. In contrast, in the BMMY medium, considerable proportion of the stylopine produced was present in the medium. At 72 h, stylopine content in the BMMY medium was 14.3-fold higher than that in BM medium (3125 µg/L in BMMY and 218 µg/L in BM medium). These results indicate that the type of medium used exerts a significant effect on stylopine production and its efflux into the medium. Considering that the efficient recovery of end-product was possible from the medium, without the extraction from cells, we considered the BMMY medium to be more appropriate for stylopine production and used this medium for further analysis.
Growth ofP. pastoris一个d (S)-stylopine production from (S)-reticuline in various media. Growth was evaluated by measuring the optical density at 600 nm (a), and the time-dependent production of (S)-stylopine inP. pastoriscells (b) and the medium (c) was determined.P. pastoriscells were cultured in each medium containing 100 µM (S)-reticuline, and sampled at the times indicated. Results indicate mean ± standard deviation of triplicate experiments
Reticuline production usingE. coliin the BMMY medium
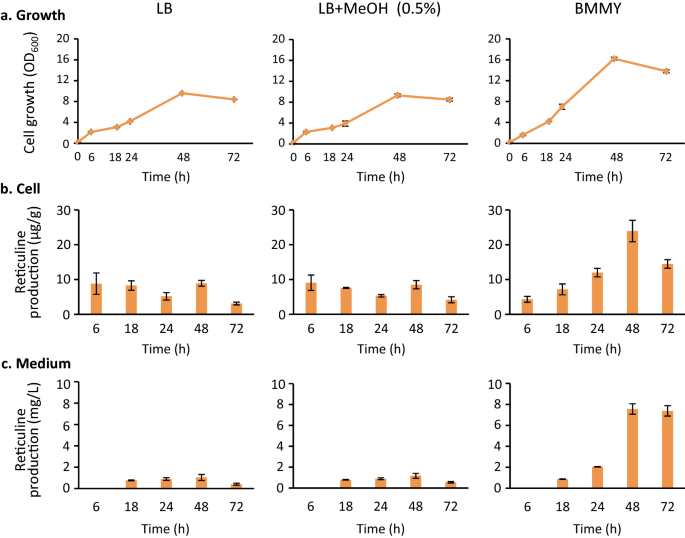
Next, we examined the effect of MeOH, which was added to the BMMY medium to induce protein expression inP. pastoris, on the growth and reticuline production in reticuline-producingE. colicells.E. colicells were pre-cultured in Luria Bertani (LB) medium, and then inoculated in either LB medium, LB medium containing MeOH, or BMMY medium, and cultured under shaking conditions (250 rpm) at 30 °C. All media contained isopropyl β-d-thiogalactopyranoside (IPTG) (0.1 mM) to induce the biosynthetic enzymes for reticuline inE. colicells. In LB medium, the addition of 0.5% MeOH exerted negligible effect on cell growth and reticuline production (Fig.3).令人惊讶的是,我更好地观察细胞生长n the BMMY medium, compared to the LB medium. Additionally, reticuline was efficiently produced in the BMMY medium. After incubation for a duration of 24 h, the cellular reticuline content in BMMY was 2 to 3.5 times higher and its concentration in BMMY medium was 2 to 13 times higher than that observed in LB medium. At 72 h, the reticuline content in the BMMY medium was found to be 7.4 mg/L, which was 13-fold higher than that observed in the LB medium containing MeOH (0.56 mg/L). Reticuline is secreted into the BMMY medium and this is desirable for efficient transfer of the biosynthetic intermediate in the co-culture system. Therefore, we selected this medium for use in the co-culture system.
Growth ofE. coli一个d (S)-reticuline production from glycerol. Growth was evaluated by measuring the optical density at 600 nm (a), and the time-dependent production of (S)-reticuline inE. colicells (b) and the medium (c) was determined.E. colicells were cultured in LB, LB containing MeOH (0.5%) or the BMMY medium. These media contained IPTG (0.1 mM), glycerol (5 g/L), and appropriate antibiotics. Cells and media were sampled at the times indicated. Results indicate mean ± standard deviation of triplicate experiments
E. coli-P. pastorisco-culture system for stylopine production
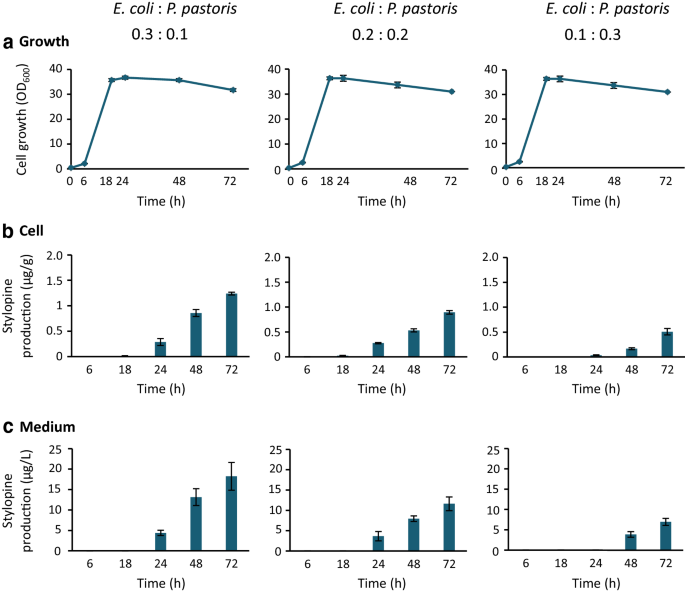
We investigated whether the co-culture of reticuline-producingE. coli一个d stylopine-producingP. pastoriscould lead to the de novo production of stylopine from a simple carbon source (Fig.1b).E. coli一个dP. pastoris细胞被pre-cultured磅和YPD媒体respectively. Both cells were co-cultured in the BMMY medium containing IPTG, MeOH, and glycerol as a simple carbon source. This culture system showed successful in de novo production of stylopine from glycerol. Therefore, we further investigated the effect of initial inoculation ratio on stylopine production.E. coli一个dP. pastoriscells inoculated at ratios of 0.3:0.1, 0.2:0.2, and 0.1:0.3, acquired at OD600, showed similar exponential cell growth up to 18 h, after which they entered the stationary phase (Fig.4a). Stylopine production was observed in all cases and was the highest in the 0.3:0.1 ratio in both the cells and the medium (Fig.4b and c); higher the ratio ofE. colicells, higher the rate of stylopine production. At 72 h, the stylopine content in the medium was found to be approximately 20 µg/L. These results indicate that the upstreamE. colistrain is the rate-limiting factor for stylopine production in this co-culture system. Consistent with this hypothesis, reticuline, an important intermediate produced byE. colicells, was undetectable at most time points in both the cells and the medium (Additional file 2: Fig. S2). The growth ofE. colicells in co-culture (Additional file2: Fig. S3) was not as well as that in a single culture (Fig.3), therefore, the higher ratio ofE. colicells would be suitable for higher production of stylopine.
De novo (S)-stylopine production from glycerol using the co-culture ofE. coli一个dP. pastoris. Reticuline-producingE. coli一个d stylopine-producingP. pastoriswere pre-cultured in LB medium containing antibiotics and YPD medium, respectively. The cultures were inoculated, at the initial ratio indicated, into the BMMY medium containing IPTG (0.1 mM), glycerol (5 g/L), and antibiotics. Growth was estimated by measuring the optical density at 600 nm (a), and the time-dependent production of (S)-stylopine in cells (b) and the medium (c) was determined. Results indicate mean ± standard deviation of triplicate experiments
As illustrated in Fig.3, reticuline production in theE. colicells was observed from 6 h, while the reticuline secreted into the medium increased after 18 h. In contrast, the induction of biosynthetic enzymes inP. pastoriscells seemed to require 18 h or more, since stylopine production was observed after 18 h even when reticuline was added to the medium at the beginning of the experiment (Fig.2).Similar conditions would be required for induction of biosynthetic enzymes and biosynthesis inP. pastorispresent in the co-culture system, since reticuline was observed in the cells and the medium only at 6 h (Additional file2: Fig. S2). For a duration of up to 18 h, the downstreamP. pastorisstrain might be the rate-limiting factor for stylopine production. The earlier induction of biosynthesis enzymes inP. pastorisduring pre-culture might accelerate the production of stylopine and relieve this limitation. In addition, successive feeding of the substrate, glycerol, in this case, might enhance the productivity, as previously reported forP. pastoris[23].
In this study, only stylopine production was investigated, using reticuline as a common intermediate. However, various compounds such as thebaine and resveratrol have been produced fromE. coli–E. coliorE. coli–S. cerevisiaeco-culture, using some common intermediates such as reticuline andp香豆交流id [7,12,13,14]. In addition, in the co-culture ofP. pastoris–P. pastoris, the production of lovastatin and stylopine was reported [19,23]. These suggest thatE. coli一个dP. pastorisare relatively able to efflux or influx the various intermediates. Therefore, the co-culture system established in this study would be applicable to the production of other valuable metabolites, through the other intermediates.
We selected a co-culture system ofE. colicells andP. pastoriscells.E. coliis suitable for the high production of some intermediates derived from central metabolites [30], i.e., reticuline andp香豆交流id, and downstreamP. pastorisis appropriate for further modification of intermediates using well expressed enzymes like BBE and P450. However, recently, higher production of reticuline was reported usingS. cerevisiae[31]. In the future, co-cultivation ofS. cerevisiae一个dP. pastorismight be useful. Since this is a co-culture of yeast cells, that is,S. cerevisiae-P. pastoris, optimization of the growth condition and productivity might be easier than co-culture ofE. coli-P. pastoris. Production of diverse valuable metabolites will become possible through the application of various co-culture systems, including the co-culture system betweenE. coli一个dP. pastorisestablished in this study.
In a co-culture system, efficient transfer of biosynthetic intermediates between cell lines is also important. We showed that the expression of an alkaloid transporter,Arabidopsis thalianaDTX1, in reticuline-producingE. colicells significantly enhanced reticuline production and its efflux into the medium [32]. Therefore, the use of this transporter-expressingE. coliin the present co-culture system may lead to enhanced production of stylopine. In a previous study, we have also shown that enhanced reticuline efflux into the medium releases the negative feedback on the biosynthetic enzymes such as methyltransferases, leading to the induction of reticuline-related biosynthesis pathways in the cells [32]. In the present co-culture, reticuline, released fromE. colicells, was quickly converted byP. pastoriscells and its concentration in the medium was low, which might have enhanced reticuline production byE. colicells. Substrate uptake byP. pastoriscells is also important. It has been reported that expression of a purine permease, BUP1, which performs the uptake of the intermediates of BIA, inS. cerevisiaeexpressing thebaine biosynthetic enzymes, significantly improves thebaine production [33]. Since this transporter showed reticuline uptake activity, expression of this transporter in stylopine-producingP. pastorismight also lead to improvement of substrate transfer and productivity. Transport engineering may also contribute to the development of a co-culture system in the future.
Conclusions
We successfully developed an大肠coli-P。pastorisco-culture platform that enabled de novo production of a valuable alkaloid, stylopine. The BMMY medium is appropriate for production and secretion of compounds into the medium in bothE. coli一个dP. pastoris. Metabolite production increased when theE. coliratio was higher in the co-culture system. The results of this study are of considerable significance sinceP. pastorisis a novel microorganism used for the industrial production of pharmaceuticals [16,17,18]. This platform can potentially lead to a low-cost and stable supply of various valuable compounds.
Methods
化学物质
(S)-Reticuline was synthesized and purified as per methods previously described [26]. (S)-Stylopine was prepared from coptisine chloride purchased from FujiFilm Wako Pure Chemical Corporation (Osaka, Japan); the preparation was achieved via chemical reduction with sodium borohydride.
Reticuline-producingEscherichia coli一个d stylopine-producing yeast cells
Reticuline-producingE. colicells (designated as the AN2104 strain) were generated by introducing four plasmids, for genes encoding reticuline biosynthetic enzymes, as per protocols described previously [32] (Additional file1: Table S1).P. pastoriscells (designated as the B52 strain), containing three genes encoding biosynthetic enzymes (BBE, CYP719A5, and CYP719A2) and enabling the production of stylopine from reticuline, were also generated as per methods described previously [23] (Additional file1: Table S1).
Stylopine production from (S)-Reticuline byP. pastoriscells in different culture media
Stylopine-producing B52 cells were grown in the YPD medium (1% yeast extract, 2% peptone, and 2% dextrose) at 30 °C under shaking conditions (200 rpm) until the achievement of an OD600of 3. The cells were then collected and resuspended in either MM (1.34% YNB, 4 × 10−5% biotin, 0.5% methanol), BMM (100 mM potassium phosphate, pH 6.0, 1.34% YNB, 4 × 10−5% biotin, 0.5% methanol), BM (0.5% yeast extract, 1% methanol), or BMMY (1% yeast extract, 2% peptone, 100 mM potassium phosphate, pH 6.0, 1.34% YNB, 4 × 10−5% biotin, 0.5% methanol) medium at an OD600of 0.6. After supplementing with 100 µM (S) -reticuline,细胞被孵化t 30 °C under shaking conditions (250 rpm) and sampled at 6, 18, 24, 48, and 72 h along with the culture medium.
Reticuline production from glycerol byE. colicells in the BMMY medium
Reticuline-producing AN2104 cells were pre-cultured overnight at 30 °C under shaking conditions (200 rpm) in LB medium containing appropriate antibiotics (2 mg/L tetracycline [Nacalai Tesque, Kyoto, Japan], 80 mg/L ampicillin [Sigma-Aldrich, St. Louis, MO, USA], 100 mg/L spectinomycin [Nacalai Tesque], and 30 mg/L chloramphenicol [Nacalai Tesque]). The overnight culture was inoculated at OD600= 0.2 in fresh LB medium containing appropriate antibiotics and the cells were grown for 2 h at 30 °C until the achievement of an OD600of 0.6. The cells were then collected and resuspended in the LB, LB containing MeOH (0.5%) or BMMY medium, containing appropriate antibiotics, IPTG (0.1 mM), and glycerol (5 g/L) with an initial OD600of 0.2, and were incubated at 30 °C under shaking conditions (250 rpm). The samples were harvested at 6, 18, 24, 48, and 72 h after induction.
De novo production of stylopine from co-culture ofE. coli一个dP. pastoriscells in the BMMY medium
Reticuline-producing AN2104 cells were pre-cultured in LB medium containing the appropriate antibiotics, as per methods described above. Stylopine-producing B52 cells were pre-cultured in YPD medium as per methods described above. Both types of pre-cultured cells were collected and resuspended in the BMMY medium containing 0.1 mM IPTG, appropriate antibiotics, and glycerol, at the initial concentration ratios of OD600as indicated earlier. The cells were incubated at 30 °C under shaking conditions (250 rpm). The samples were harvested at 6, 18, 24, 48, and 72 h after induction.
Metabolite analysis
All culture samples were centrifuged and separated into supernatants (medium) and pellets (cells). Trichloroacetate (2% final concentration) was added to the supernatant to precipitate the proteins, followed by centrifugation at 15,000×gfor 20 min. The pellets were subjected to washing steps with ice water and incubated overnight with 40 µL/mg fresh weight (FW) (forE. coli) or 20 µL/mg FW (forP. pastoris) methanol containing 0.01 N HCl. These samples were then centrifuged at 15,000×gfor 15 min, and the supernatants obtained thereafter were used for analysis.
All samples were filtered using 0.45 μm Cosmospin Filters (Nacalai Tesque), and analyzed by conducting UPLC-MS using the ACQUITY UPLC system with QDa mass detector (Waters Corp., Milford, MA, USA); mobile phase comprised 0.01% (v/v) acetic acid in water (solvent A) and 0.01% (v/v) acetic acid in acetonitrile (solvent B). Alkaloids were separated via gradient elution as follows: mobile phase was subjected to linear decrease from 95% A to 60% A in 9 min, following decrease from 60% A to 50% A in 3 min, and increase from 50% A to 95% A in 3 min; column, CORTECS UPLC C18 (1.6 μm, 2.1 × 100 mm; Waters Corp.) was used considering temperature of 40 °C with a flow rate of 0.3 mL/min.
The QDa conditions were set as follows: cone voltage, 15 V; capillary voltage, 0.8 kV; and source temperature, 600 °C. Reticuline (m/z = 330), and stylopine (m/z = 324) were detected using the single-ion recording (SIR) mode, and each peak was identified by conducting direct comparison with peaks corresponding to authentic standard chemicals. The content of each alkaloid was quantified using a standard curve.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rischer H, Häkkinen ST, Ritala A, Seppänen-Laakso T, Miralpeix B, Capell T, et al. Plant cells as pharmaceutical factories. Curr Pharm Des. 2013;19:5640–60.
Cravens A, Payne J, Smolke CD. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat Commun. 2019;10:2142.
Yang D, Park SY, Park YS, Eun H, Lee SY. Metabolic engineering ofEscherichia colifor natural product biosynthesis. Trends Biotechnol. 2020;38:745–65.
Nakagawa A, Matsumura E, Koyanagi T, Katayama T, Kawano N, Yoshimatsu K, et al. Total biosynthesis of opiates by stepwise fermentation using engineeredEscherichia coli. Nat Commun. 2016;7:10390.
Galanie S, Thodey K, Trenchard IJ, Filsinger Interrante M, Smolke CD. Complete biosynthesis of opioids in yeast. Science. 2015;349:1095–100.
Srinivasan P, Smolke CD. Biosynthesis of medicinal tropane alkaloids in yeast. Nature. 2020;585:614–9.
Yuan SF, Yi X, Johnston TG, Alper HS. De novo resveratrol production through modular engineering of anEscherichia coli-Saccharomyces cerevisiaeco-culture. Microb Cell Fact. 2020;19:143.
Croteau R, Kutchan TM, Lewis NG, Buchanan B, Gruissem W, Jones R, editors. Natural products (secondary metabolites). Biochemistry & molecular biology of plants. Maryland: American Society of Plant Physiologists; 2000. pp. 1250–318.
Zhang H, Wang X. Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng. 2016;37:114–21.
Jawed K, Yazdani SS, Koffas MA. Advances in the development and application of microbial consortia for metabolic engineering. Metab Eng Commun. 2019;9:e00095.
McCarty NS, Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019;37:181–97.
Wang X, Li Z, Policarpio L, Koffas MAG, Zhang H. De novo biosynthesis of complex natural product sakuranetin using modular co-culture engineering. Appl Microbiol Biotechnol. 2020;104:4849–61.
Jones JA, Vernacchio VR, Collins SM, Shirke AN, Xiu Y, Englaender JA, et al. Complete biosynthesis of anthocyanins usingE. coliPolycultures. mBio. 2017;8:e00621.
Minami H, Kim JS, Ikezawa N, Takemura T, Katayama T, Kumagai H, et al. Microbial production of plant benzylisoquinoline alkaloids. Proc Natl Acad Sci U S A. 2008;105:7393–8.
Sgobba E, Stumpf AK, Vortmann M, Jagmann N, Krehenbrink M, Dirks-Hofmeister ME, et al. SyntheticEscherichia coli-Corynebacterium glutamicumconsortia for l-lysine production from starch and sucrose. Bioresour Technol. 2018;260:302–10.
Vogl T, Hartner FS, Glieder A. New opportunities by synthetic biology for biopharmaceutical production inPichia pastoris. Curr Opin Biotechnol. 2013;24:1094–101.
Schwarzhans JP, Luttermann T, Geier M, Kalinowski J, Friehs K. Towards systems metabolic engineering inPichia pastoris. Biotechnol Adv. 2017;35:681–710.
Yang Z, Zhang Z. Engineering strategies for enhanced production of protein and bio-products inPichia pastoris: A review. Biotechnol Adv. 2018;36:182–95.
Liu Y, Tu X, Xu Q, Bai C, Kong C, Liu Q, et al. Engineered monoculture and co-culture of methylotrophic yeast for de novo production of monacolin J and lovastatin from methanol. Metab Eng. 2018;45:189–99.
Liu XB, Liu M, Tao XY, Zhang ZX, Wang FQ, Wei DZ. Metabolic engineering ofPichia pastorisfor the production of dammarenediol-II. J Biotechnol. 2015;216:47–55.
Wriessnegger T, Augustin P, Engleder M, Leitner E, Müller M, Kaluzna I, et al. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering ofPichia pastoris. Metab Eng. 2014;24:18–29.
Moser S, Strohmeier GA, Leitner E, Plocek TJ, Vanhessche K, Pichler H. Whole-cell (+)-ambrein production in the yeastPichia pastoris. Metab Eng Commun. 2018;7:e00077.
Hori K, Okano S, Sato F. Efficient microbial production of stylopine using aPichia pastorisexpression system. Sci Rep. 2016;6:22201.
Minami H. Fermentative production of plant benzylisoquinoline alkaloids in microbes. Biosci Biotechnol Biochem. 2013;77:1617–22.
Diamond A, Desgagné-Penix I. Metabolic engineering for the production of plant isoquinoline alkaloids. Plant Biotechnol J. 2016;14:1319–28.
Matsumura E, Nakagawa A, Tomabechi Y, Ikushiro S, Sakaki T, Katayama T, et al. Microbial production of novel sulphated alkaloids for drug discovery. Sci Rep. 2018;8:7980.
Meng Y, Shao X, Wang Y, Li Y, Zheng X, Wei G, et al. Extension of cell membrane boosting squalene production in the engineeredEscherichia coli. Biotechnol Bioeng. 2020;117:3499–507.
Jang SI, Kim BH, Lee WY, An SJ, Choi HG, Jeon BH, et al. Stylopine from Chelidonium majus inhibits LPS-induced inflammatory mediators in RAW 264.7 cells. Arch Pharm Res. 2004;27:923–9.
Okamoto T, Kawaguchi K, Watanabe S, Agustina R, Ikejima T, Ikeda K, et al. Characterization of human ATP-binding cassette protein subfamily D reconstituted into proteoliposomes. Biochem Biophys Res Commun. 2018;496:1122–7.
Pyne ME, Narcross L, Martin VJJ. Engineering plant secondary metabolism in microbial systems. Plant Physiol. 2019;179:844–61.
Pyne ME, Kevvai K, Grewal PS, Narcross L, Choi B, Bourgeois L, et al. A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids. Nat Commun. 2020;11:3337.
Yamada Y, Urui M, Oki H, Inoue K, Matsui H, Ikeda Y, et al. Transport engineering for improving production and secretion of valuable alkaloids inEscherichia coli. Metab Eng Commun. 2021;13:e00184.
Dastmalchi M, Chang L, Chen R, Yu L, Chen X, Hagel JM, et al. Purine permease-type benzylisoquinoline alkaloid transporters in opium poppy. Plant Physiol. 2019;181:916–33.
Acknowledgements
We thank Ms. Yoko Nakahara (Kobe Pharmaceutical University, Japan) for provision of assistance with the experiments.
Funding
This work was supported by JSPS KAKENHI (Grant Number 17H05453 to N.S.), Grant-in-Aid for Scientific Research on Innovative Areas.
Author information
Authors and Affiliations
Contributions
MU, YY, FS, HM and NS designed the experiments. MU, YY, YI, AN and NS performed the experiments. MU, YY, YI, FS, HM and NS analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
关于文书期刊上施普林格自然保持中立isdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Plasmids used in this study.
Additional file 2: Fig. S1.
Detection of (S)-stylopine in various media. Single-ion chromatogram of (S)-stylopine inP. pastoriscells grown in each medium and authentic standard. N.D.; not detected.Fig. S2(S)-Reticuline production in the co-culture ofE. coli一个dP. pastoris. Cells were cultured as per methods described in the Fig.4legend. (S)-Reticuline in the cells (a) and medium (b) were detected and quantified. Results indicate mean ± standard deviation of triplicate experiments.Fig. S3Growth and cell density ofE. coli一个dP. pastorisin co-culture system. An initial inoculation ratio ofE. coli and P. pastoriscells was 0.3:0.1. Growth was evaluated by measuring the optical density at 600 nm (a). The number ofE. colicells (b) andP. pastoriscells (c) were counted using a bacteria counting chamber and a microscope. Results indicate mean ± standard deviation of technical triplicates.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Urui, M., Yamada, Y., Ikeda, Y.et al.Establishment of a co-culture system usingEscherichia coli一个dPichia pastoris(Komagataella phaffii) for valuable alkaloid production.Microb Cell Fact20, 200 (2021). https://doi.org/10.1186/s12934-021-01687-z
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12934-021-01687-z