- Research
- Open access
- Published:
Effects ofMIG1,TUP1andSSN6deletion on maltose metabolism and leavening ability of baker’s yeast in lean dough
188bet亚洲体育平台volume13, Article number:93(2014)
Abstract
Background
Glucose repression is a global regulatory system in baker’s yeast. Maltose metabolism in baker’s yeast strains is negatively influenced by glucose, thereby affecting metabolite productivity (leavening ability in lean dough). Even if the general repression system constituted byMIG1,TUP1andSSN6factors has already been reported, the functions of these three genes in maltose metabolism remain unclear. In this work, we explored the effects ofMIG1and/orTUP1and/orSSN6deletion on the alleviation of glucose-repression to promote maltose metabolism and leavening ability of baker’s yeast.
Results
Results strongly suggest that the deletion ofMIG1and/orTUP1and/orSSN6can exert various effects on glucose repression for maltose metabolism. The deletion ofTUP1was negative for glucose derepression to facilitate the maltose metabolism. By contrast, the deletion ofMIG1and/orSSN6, rather than other double-gene or triple-gene mutations could partly relieve glucose repression, thereby promoting maltose metabolism and the leavening ability of baker’s yeast in lean dough.
Conclusions
The mutants of industrial baker’s yeast with enhanced maltose metabolism and leavening ability in lean dough were developed by genetic engineering. These baker’s yeast strains had excellent potential industrial applications.
Background
Baker’s yeast (Saccharomyces cerevisiae) is the key microorganism used in the baking industry. Although a small amount of free sugars exists in lean dough with no added sugar, maltose represents the principal source of fermentable carbon during dough fermentation [[118博金宝那个真网 ]–[118博金宝那个真网 ]]。A good baker’s yeast should rapidly ferment maltose. However, glucose and fructose are the first sugars to be used during fermentation, and the presence or uptake of glucose has a negative impact on the metabolism of other carbon sources [[118博金宝那个真网 ]–[118博金宝那个真网 ]]。Given that the genes involved in maltose utilization are repressed by glucose, a reasonable way to improve maltose metabolism and leavening ability of baker’s yeast is by effectively alleviating glucose repression.
Mig1, a Cys2His2zinc-finger protein, binds to the promoters of several genes and represses their transcription when glucose is added to the medium [[118博金宝那个真网 ]–[118博金宝那个真网 ]]。Hu et al. have shown that Mig1p represses the transcription of all threeMALgenes essential to maltose metabolism by binding the upstream genes [[118博金宝那个真网 ]]。In addition, Mig1 inhibits transcription by recruiting the general co-repressor complex Ssn6-Tup1 [[118博金宝那个真网 ]]。Ssn6-Tup1 is one of the first co-repressor complexes to be identified. As with other co-repressors, the specificity of repression is determined by sequence-specific DNA binding repressors, which recruit Ssn6-Tup1 to the target gene promoters; these repressors include Mig1 [[118博金宝那个真网 ]–[118博金宝那个真网 ]]。Therefore, a strong correlation amongMIG1,TUP1andSSN6for glucose repression was observed. Previous studies have shown that the maltose metabolism of baker’s yeast could be partly glucose derepressed byMIG1single-gene mutant through enhancing the transcription of theMALgene [[118博金宝那个真网 ]–[118博金宝那个真网 ]]。However, the effect of relieving glucose repression on maltose metabolism of baker’s yeast by silencingTUP1and/orSSN6remains unclear. Furthermore, maltose metabolism of baker’s yeast through combination mutations ofMIG1,TUP1andSSN6, which breaking the regulatory pathway of glucose repression, remains unclear.
In this study, we disrupted the regulatory pathway of glucose repression by deletingMIG1and/orTUP1and/orSSN6to investigate the effects ofMIG1,TUP1andSSN6在maltose metabolism and leavening ability of baker’s yeast. The results explicitly suggest that the deletion of theMIG1and/orTUP1and/orSSN6genes lead to different results in the tested conditions. Deletion ofMIG1and/orSSN6is more efficient thanTUP1deletion and other combination deletions ofMIG1,TUP1andSSN6在glucose derepression for maltose metabolism and leavening ability of baker’s yeast in lean dough. This finding lays a foundation for the optimization of industrial baker’s yeast strains.
Results
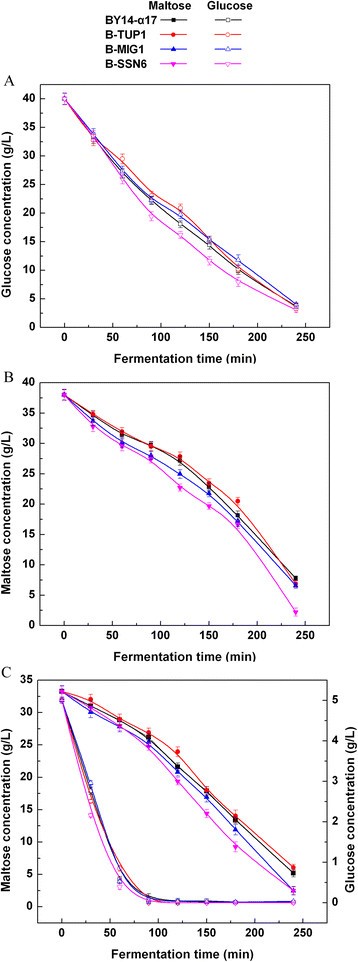
Sugar consumption of single-gene deletion strains in low sugar model liquid dough (LSMLD) medium
The impact of single-gene mutation ofMIG1,TUP1andSSN6在sugar consumption was assayed in three LSMLD media. TheTUP1single-gene-deletion strain B-TUP1 cannot rapidly utilize maltose and is inferior to the parental strain BY14-α17, when glucose was exhausted in the glucose-maltose LSMLD medium (Figure118博金宝那个真网 C). Compared with the parental strain BY14-α17, theMIG1single-gene-deletion strain B-MIG1 did not evidently change in glucose and maltose LSMLD media (Figures118博金宝那个真网 A to B). However, compared with the parental strain, a 10.8% increase of maltose utilization efficiency (21.3% in the parental strain and 23.6% in the strain B-MIG1,P < 0.05) of the strain B-MIG1 was observed in glucose-maltose LSMLD medium, when glucose was exhausted (Figure118博金宝那个真网 C). Simultaneously, the time span between the point when half of the glucose and that of the maltose had been consumed decreased by 10.2% compared with the parental strain (Table118博金宝那个真网 ). The utilization efficiency of sugar distinctly increased in theSSN6single-gene-deletion strain B-SSN6 compared with the parental strain BY14-α17. The maltose utilization efficiency in B-SSN6 was 18.3% and 19.7% higher than that of the parental strain in maltose (79.6% in the parental strain and 94.2% in the strain B-SSN6,P < 0.05) and glucose-maltose (21.3% in the parental strain and 25.5% in the strain B-SSN6,P < 0.05) LSMLD media, respectively (Figures118博金宝那个真网 B to C). Furthermore, compared with the parental strain BY14-α17, the time span in B-SSN6 decreased from 2.15 h to 1.76 h (Table118博金宝那个真网 ).
Concentration of residual sugar in parental strain and single-gene mutants in LSMLD medium.Fresh yeast cells were inoculated into(A)glucose LSMLD medium,(B)maltose LSMLD medium and(C)glucose-maltose LSMLD medium, and were sampled at suitable intervals. Data are average of three independent experiments and error bars represent ± SD.
These results demonstrate that the single-gene deletion of the three genes (MIG1,TUP1andSSN6) resulted in different effects on the alleviation of glucose repression in the maltose utilization of baker’s yeast. Single-gene deletions ofSSN6andMIG1promote the glucose derepression. Particularly, the single-gene deletion ofSSN6was more effective than theMIG1single-gene deletion. However,TUP1single-gene deletion was negative to relieve glucose repression to promote the maltose metabolism.
Sugar consumption of double-gene deletion strains in LSMLD medium
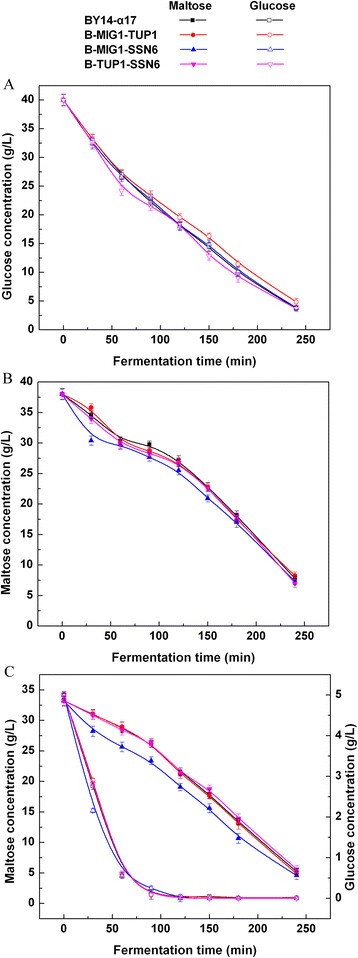
The maltose metabolism was tested for the double-gene mutants ofMIG1,TUP1andSSN6in the LSMLD medium. Although glucose and maltose decreased with BY14-α17 in the strains B-MIG1-TUP1 and B-TUP1-SSN6 in the three LSMLD media (Figure118博金宝那个真网 ), the time span of B-TUP1-SSN6 was still 6.98% higher than the parental strain (Table118博金宝那个真网 ). By contrast, a positive effect with decreased time span (10.7%) was obtained in B-MIG1-SSN6 (Table118博金宝那个真网 ). When the yeast cells were inoculated in the maltose and the glucose-maltose LSMLD media, the strain B-MIG1-SSN6 exhibited a substantially more rapid sugar-uptake than the other strains. Compared with the parental strain BY14-α17, maltose utilization efficiency (21.3% in the parental strain and 29.7% in the strain B-MIG1-SSN6,P < 0.05) distinctly increased by 39.4% in the strain B-MIG1-SSN6, when glucose was exhausted in the glucose-maltose LSMLD medium (Figure118博金宝那个真网 C).
Concentration of residual sugar in parental strain and double-gene mutants in LSMLD medium.Fresh yeast cells were inoculated into(A)glucose LSMLD medium,(B)maltose LSMLD medium and(C)glucose-maltose LSMLD medium, and sampled at suitable intervals. Data are average of three independent experiments and error bars represent ± SD.
These results indicate that the double-gene deletion of the three genes (MIG1,TUP1andSSN6) also generated different effects on the maltose metabolism of baker’s yeast by alleviating glucose repression. The co-gene-deletion ofMIG1andSSN6mitigated glucose repression, which is more efficient thanMIG1-TUP1andTUP1-SSN6double-gene deletions with no evident function in maltose metabolism.
Sugar consumption of the triple-gene deletion strain in LSMLD medium
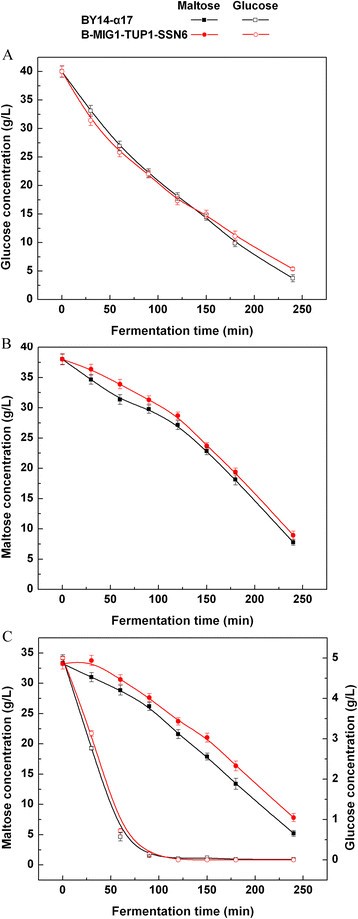
The maltose metabolism was further investigated with B-MIG1-TUP1-SSN6, which performs the triple-gene-deletion ofMIG1,TUP1andSSN6. Surprisingly, in B-MIG1-TUP1-SSN6, the maltose uptake was considerably delayed compared with the parental strain BY14-α17 until the termination of the process in the maltose LSMLD medium (Figure118博金宝那个真网 B). Compared with the parental strain, the maltose utilization efficiency (21.3% in the parental strain and 16.9% in the strain B-MIG1-TUP1-SSN6,P < 0.05) of the strain B-MIG1-TUP1-SSN6 decreased by 20.7% in the glucose-maltose LSMLD medium (Figure118博金宝那个真网 C). The consumption of maltose was slower than BY14-α17 throughout the process. Moreover, the time span was evidently increased (from 2.15 h to 2.32 h) (Table118博金宝那个真网 ).
Concentration of residual sugar in parental strain and triple-gene mutants in LSMLD medium.Fresh yeast cells were inoculated into(A)glucose LSMLD medium,(B)maltose LSMLD medium and(C)glucose-maltose LSMLD medium, and sampled at suitable intervals. Data are average of three independent experiments and error bars represent ± SD.
These results suggest that theMIG1,TUP1andSSN6triple-gene deletions were unavailable to relieve glucose repression and enhance maltose metabolism of baker’s yeast, thoughMIG1,TUP1andSSN6could function as a complex that affects the glucose-repressible genes [[118博金宝那个真网 ]]。
Growth and fermentation properties
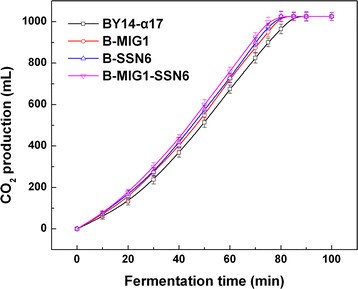
Considering the diversity of sugars consumption of the eight strains obtained, we further investigated the growth characteristics (specific growth rate and biomass yield) under different carbon sources and explored the leavening ability in lean dough. The specific growth rate and biomass yield of the single-gene-deletion and double-gene-deletion strains illustrated in Table118博金宝那个真网 comparatively remained stable (small difference with no statistical significance), revealing that the single-gene and double-gene deletions ofMIG1,TUP1andSSN6did not influence the growth of the strains. However, the specific growth rate of the triple-gene-deletion B-MIG1-TUP1-SSN6 (0.14 h−1) was lower than that of the parental strain BY14-α17 (0.19 h−1) in the maltose LSMLD medium. Compared with the parental strain, the biomass yield of B-MIG1-TUP1-SSN6 decreased from 5.9 g/L to 5.1 g/L in the glucose-maltose LSMLD medium (Table118博金宝那个真网 ). The positive mutants B-MIG1, B-SSN6 and B-MIG1-SSN6 performed well for the leavening ability. Compared with the parental strain BY14-α17, the amount of evolved CO2by B-MIG1, B-SSN6 and B-MIG1-SSN6 within 70 min increased from 825 mL to 875 mL (P < 0.05), 900 mL (P < 0.01) and 925 mL (P < 0.01), respectively (Figure118博金宝那个真网 ), while the other strains (B-TUP1, B-MIG1-TUP1, B-TUP1-SSN6 and B-MIG1-TUP1-SSN6) showed lower CO2production (data not shown). Moreover, the fermentation time in B-MIG1, B-SSN6 and B-MIG1-SSN6 were evidently shortened, compared with the parental strain.
These fermentation findings directly correspond with sugar consumption in the LSMLD medium suggesting that the deletion ofMIG1and/orTUP1and/orSSN6led to different effects on the leavening ability of baker’s yeast in lean dough. Particularly,MIG1and/orSSN6deletions could improve the fermentation with stable physiological characteristics.
Discussion
Glucose has dramatic down-regulating effects on the metabolism of other sugars and on the leavening properties of baker’s yeast strains. Glucose does not repress all glucose-repressible genes in a similar manner [[118博金宝那个真网 ]]。Numerous reports have shown that the Mig1 repressor and Ssn6-Tup1 co-repressor are central components of the glucose repression machinery involved in the regulation ofMAL表达式[[118博金宝那个真网 ],[118博金宝那个真网 ],[118博金宝那个真网 ]]。Moreover,MIG1deletion does not alleviate glucose repression of maltose utilization [[118博金宝那个真网 ],[118博金宝那个真网 ]]。Probably, the genetic backgrounds of theS. cerevisiaestrains used led to the differences in maltose consumptions of the cells.TUP1andSSN6mutants produce various phenotypes, including constitutive derepression of numerous glucose-repressible genes, calcium-dependent flocculation, mating-type defects in MATα cells and non-sporulation of homozygous diploids [[118博金宝那个真网 ],[118博金宝那个真网 ]]。However, the different combinations of mutatedMIG1,TUP1andSSN6have never been conceived to improve the maltose metabolism. In this study, the deletion mutations ofMIG1and/orTUP1and/orSSN6were established for the industrial baker’s yeast cells, explicitly demonstrating that the deletion ofMIG1and/orTUP1and/orSSN6presented different effects on the maltose metabolism and leavening ability of baker’s yeast.
Compared with the parental strain, glucose repression of maltose metabolism was partly alleviated byMIG1single-gene deletion in B-MIG1 strain (Figures118博金宝那个真网 B to C), supporting the point that the disruption ofMIG1causes partial alleviation of glucose repression by the secreted metabolites [[118博金宝那个真网 ],[118博金宝那个真网 ]]。Surprisingly, an apparent difference was observed between the two members of the co-repressor Ssn6-Tup1,SSN6andTUP1. The trend for sugar consumption suggests that the maltose metabolism inTUP1single-gene-deletion strain B-TUP1 was longer than the parental strain BY14-α17, pointing to a negative alleviation of glucose repression. In contrast, glucose repression was partially relieved inSSN6single-gene-deletion strain B-SSN6, increasing maltose metabolism (Figures118博金宝那个真网 B to C). The different functional domains of Tup1 and Ssn6 involved in glucose control are probably the major causes of the different effects of the two gene deletions on glucose repression. The functional domains of Ssn6 primarily consist of 10 tandem copies of a TPR motif and are specifically necessary for the repression of glucose-regulated genes [[118博金宝那个真网 ]]。Different domains of Tup1 can cause the repression of different target genes. For example, some WD motifs or N-terminus domains of Tup1 are not essential for repression of genes regulated by glucose [[118博金宝那个真网 ]–[118博金宝那个真网 ]]。However, certain regions of Tup1 could be necessary for the high-level expression of glucose-repressed genes, such asGALgenes for galactose fermentation [[118博金宝那个真网 ]]。Thus, we propose that the regions of Tup1 crucial to the expression ofMALgenes are disrupted through complete gene deletion. Therefore,MIG1single-gene deletion andSSN6single-gene deletion were considered effective in improving maltose metabolism in the industrial baker’s yeast.
The combined effects ofMIG1,SSN6andTUP1在the maltose metabolism in industrial baker’s yeast were further investigated. Combined mutations ofTUP1withMIG1orSSN6compensated for the slow maltose metabolism of the strain B-TUP1, while the rates of maltose consumption in the mutants B-MIG1-TUP1 and B-TUP1-SSN6 were only close to that of the parental strain BY14-α17 (Figure118博金宝那个真网 ).TUP1single-gene deletion is possible in its negative effect limited to the alleviation of glucose repression. Hence,MIG1orSSN6deletion withTUP1cannot enhance the maltose metabolism. The double-gene mutant strain B-MIG1-SSN6 was less glucose repressed compared with the parental strain (Figure118博金宝那个真网 C). This finding corresponds with the studies, which showed that the interactions with DNA-binding repressors are mainly mediated through the different surfaces of Ssn6, and that Ssn6 specifically interacts with Mig1 [[118博金宝那个真网 ],[118博金宝那个真网 ]]。减轻的葡萄糖repr不足sion in B-MIG1-SSN6 could result from the co-action of Mig1 and Ssn6. Although Mig1 is unessential for tethering Ssn6 to theMALupstream, it is important for Ssn6-mediated repression in response to glucose. Surprisingly, the triple-gene mutant B-MIG1-TUP1-SSN6 was more glucose repressed than the parental strain (Figure118博金宝那个真网 ). Considering that the interactions of Ssn6-Tup1 complex contain diverse mechanisms, other mechanisms affecting theMALgenes expression are also possibly involved in the regulation of the repression by the Mig1-Tup1-Ssn6 complex [[118博金宝那个真网 ],[118博金宝那个真网 ]]。此外,糖吸收B-MI低劣G1-TUP1-SSN6 could be caused by the feeble physiological characteristic compared with the parental strain (Table118博金宝那个真网 ).
Single-gene and double-gene deletions did not present any evident changes in the specific growth rate and biomass yield in the three LSMLD media (Table118博金宝那个真网 ). In other words, the growth properties of single-gene and double-gene deletions ofMIG1and/orTUP1and/orSSN6with no distinctive difference (no statistical significance) were insufficient to affect the maltose metabolism. Therefore, the differences in maltose metabolism (maltose utilization and CO2production) do not have strong correlations with the indistinct differences in the physiological effects of the baker’s yeast in this study.
The effective alleviation of glucose repression or the rapid transition from glucose to maltose metabolism is essential to improve the leavening ability of baker’s yeast in lean dough. The single-geneMIG1/SSN6and co-gene-deletions ofMIG1andSSN6decreased the span time in the glucose-maltose LSMLD medium with stable growth properties (Tables118博金宝那个真网 and118博金宝那个真网 ). Therefore, these deletions could cause efficient leavening ability (Figure118博金宝那个真网 ). Furthermore, evident increase of the leavening ability level was observed in B-MIG1-SSN6, indicating that Mig1 and Ssn6 collectively act for the inhibition of maltose-utilizing genes. Thus, co-gene deletion ofMIG1andSSN6could significantly enhance leavening ability of baker’s yeast. These advantages are consistent with the requirement for the leavening ability of an industrial baker’s yeast strain. With minimal transformation,SSN6orMIG1single-gene deletion is necessary to obtain a baker’s yeast strain with rapid maltose metabolism.
Conclusion
The results of this study show that the glucose repression involved in the maltose metabolism can be modulated at different levels through the different mutations ofMIG1and/orTUP1and/orSSN6. The deletion ofTUP1was negative to alleviate glucose repression to facilitate the maltose metabolism. In contrast, deletions ofMIG1and/orSSN6were efficient to relieve glucose repression, therefore, promoting maltose metabolism and the leavening ability of baker’s yeast in lean dough. Hence, such baker’s yeast has excellent commercial and industrial applications.
Materials and methods
Strains and vectors
Table118博金宝那个真网 summarizes the genetic properties of all strains and plasmids used in this study.
Growth, cultivation and fermentation conditions
Recombinant DNA was amplified inEscherichia coliDH5α, which was grown at 37°C in Luria–Bertani medium (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) supplemented with 100 μg/mL ampicillin. The plasmid was obtained using a Plasmid Mini Kit II (D6945, Omega, USA).
The yeast strains were maintained in yeast extract peptone dextrose (YEPD) medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose) at 30°C. Over the next enrichment of the molasses medium, cells were harvested through centrifugation (4°C, 1500 ×g, 5 min) and were washed twice with sterile water at 4°C in the succeeding fermentation experiments. To investigate the degree of repression between the three repression factors under different concentrations of extracellular maltose, we used the low sugar model liquid dough fermentation medium [LSMLD fermentation medium, 2.5 g/L (NH4)2SO4, 5 g/L urea, 16 g/L KH2PO6, 5 g/L Na2HPO4, 0.6 g/L MgSO4, 0.0225 g / L烟酸,0.005 g / L Ca-pantothenate, 0.0025 g/L thiamine, 0.00125 g/L pyridoxine, 0.001 g/L riboflavin, and 0.0005 g/L folic acid], containing one of the three specified carbon sources (40 g/L glucose, 38 g/L maltose, and 33.25 g/L maltose mixed with 5 g/L glucose).
To select Zeocin-resistant yeast strains, 500 mg/L Zeocin (Promega, Madison, United States) was added to the YEPD plates for the yeast culture. Then, the YEPG medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L galactose) was used forCreexpression in the yeast transformants.
Construction of plasmid and yeast transformants
Genomic yeast DNA was prepared from the industrial baker’s yeast strain BY14-α17 using a yeast DNA kit (D3370-01, Omega, USA). The PCR primers used in this work are listed in Table118博金宝那个真网 .
An upstream homologous fragment of theTUP1gene was amplified by PCR using BY14-α17 genomic DNA as template with TA-U and TA-D primers. A downstream homologous fragment was similarly amplified using TB-U and TB-D primers. Then, the PCR products were digested using the appropriate endonucleases and were cloned to the pUC19 cloning vector atEcoRI andKpnI sites, andSalI andSphI sites, respectively, to construct plasmid pUC-AtBt. TheKanMX盒,聚合酶链反应使用pUG6放大the template with the primers Kan-U and Kan-D, was cloned to construct pUC-AtBtafter being digested with the appropriate endonucleases to produce the final plasmid, which was designated as pUC-KAtBt. Based on the aforementioned strategy, the pUC-KAsBsplasmid was constructed by inserting the upstream homologous fragmentSSN6A,KanMXcassette, and downstream homologous fragmentSSN6B into the pUC19 cloning vector.
Baker’s yeast transformation was achieved through lithium acetate/PEG method [[118博金宝那个真网 ]]。The deletion cassette ofTUP1A-loxP-KanMX-loxP-TUP1B是放大,变成了工业l baker’s yeast BY14-α17. The fragment was integrated into the chromosome at theTUP1locus of BY14-α17 by homologous recombination to construct theTUP1deletion strain. The selection ofTUP1deletion strain was performed using the YEPD medium supplemented with 800 mg/L G418. After selection, recombinant strains were verified with the primers listed in Table118博金宝那个真网 . Cre recombinase was expressed andKanMXwas excised after introducing the plasmid pSH-Zeocin into theTUP1deletion strain, thus resulting in B-TUP1. The same procedure was utilized to construct B-MIG1 and B-SSN6. Based on the aforementioned strategy, the double-gene mutation strains B-MIG1-TUP1, B-MIG1-SSN6 and B-TUP1-SSN6 were constructed by transforming the second deletion cassette into the single-gene mutation strains. The triple-gene mutation strain B-MIG1-TUP1-SSN6 was constructed by transformingSSN6A-loxP-KanMX-loxP-SSN6B into B-MIG1-TUP1. Finally, all of the transformants were verified through PCR with the primers listed in Table118博金宝那个真网 .
Determination of specific growth rate and biomass yield
After incubating for 24 h, the mixtures of cell culture and medium were mixed in a specific pore plate in appropriate proportions, and the growth curve was detected using bioscreen automated growth curves (Type Bioscreen C, Finland). The specific growth rate was determined with the ratio of the growth velocity to cell concentration.
Nitrocellulose filters with a pore size of 0.45 mm (Gelman Sciences, Ann Arbor, MI, USA) were pre-dried in a microwave oven at 150 W for 10 min and were subsequently weighed. Harvested cells were obtained from 10 mL of cell culture, washed twice with isometric distilled water, and dried at 105°C for 24 h. The biomass yield was determined from the slopes of the plots of biomass dry weight versus the amount of consumed sugar during exponential growth. Experiments were conducted at least thrice.
Determination of leavening ability
The leavening ability of yeast cells was assayed by measuring the CO2production in lean dough. Lean dough consisted of 280 g of flour, 150 mL of water, 4 g of salt, and 8 g of fresh yeast. The dough was evenly and quickly mixed for 5 min at 30 ± 0.2°C, and placed inside the box of a fermentograph (Type JM451, Sweden). CO2production was recorded at 30°C. Experiments were conducted at least thrice.
Analysis of sugar consumption
For extracellular sugar measurements, cultures were sampled at 30°C at suitable intervals for 4 h. The maltose content was measured through 3,5-dinitrosalicylic acid method (DNS). HPLC with a refractive index detector and an Aminex® HPX-87H column (Bio-Rad, Hercules, CA, USA) was utilized at 65°C with 5 mM H2SO4as the mobile phase at a flow rate of 0.6 mL/min [[118博金宝那个真网 ]分析了混合糖透过0。45 μm pore size cellulose acetate filters (Mil-lipore Corp, Danvers, MA, USA). The maltose utilization efficiency in maltose LSMLD medium was determined by the ratio of the consumed maltose in 240 min and the total maltose. The maltose utilization efficiency in glucose-maltose LSMLD medium was determined by the ratio of the consumed maltose, when glucose was exhausted, and the total maltose. Based on the consumption curves of glucose and maltose in the glucose-maltose LSMLD medium, the time span between the point when half of the glucose and that of the maltose had been consumed was determined. Experiments were conducted at least thrice.
Statistical analysis
Data were expressed as mean ± SD, and were accompanied by the number of experiments independently performed. The differences of the transformants compared with the parental strain were confirmed by Student’st-test. Differences atP <0.05 were considered significant differences in statistics.
References
Cousseau FE, Alves SL, Trichez D, Stambuk BU: Characterization of maltotriose transporters from theSaccharomyces eubayanussubgenome of the hybridSaccharomyces pastorianuslager brewing yeast strain Weihenstephan 34/70. Lett Appl Microbiol. 2013, 56: 21-29. 10.1111/lam.12011.
Rincón AM, Codón AC, Castrejón F, Benítez T: Improved properties of baker’s yeast mutants resistant to 2-deoxy-d-glucose. Appl Environ Microbiol. 2001, 67: 4279-4285. 10.1128/AEM.67.9.4279-4285.2001.
Jiang TX, Xiao DG, Gao Q: Characterisation of maltose metabolism in lean dough by lagging and non-lagging baker’s yeast strains. Ann Microbiol. 2008, 58: 655-660. 10.1007/BF03175571.
Horák J: Regulations of sugar transporters: insights from yeast. Curr Genet. 2013, 59: 1-31. 10.1007/s00294-013-0388-8.
Sanz P, Alms GR, Haystead TA, Carlson M: Regulatory interactions between the Reg1-Glc7 protein phosphatase and the Snf1 protein kinase. Mol Cell Biol. 2000, 20: 1321-1328. 10.1128/MCB.20.4.1321-1328.2000.
Klein CJ, Olsson L, Nielsen J: Glucose control inSaccharomyces cerevisiae: the role of Mig1 in metabolic functions. Microbiology. 1998, 144: 13-24. 10.1099/00221287-144-1-13.
Verstrepen KJ, Iserentant D, Malcorps P, Derdelinckx G, Van Dijck P, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR: Glucose and sucrose: hazardous fast-food for industrial yeast?. Trends Biotechnol. 2004, 22: 531-537. 10.1016/j.tibtech.2004.08.001.
Cao H, Yue M, Li S, Bai X, Zhao X, Du Y: The impact ofMIG1and/orMIG2disruption on aerobic metabolism of succinate dehydrogenase negativeSaccharomyces cerevisiae. Appl Microbiol Biotechnol. 2011, 89: 733-738. 10.1007/s00253-010-2894-7.
De Vit MJ, Waddle JA, Johnston M: Regulated nuclear translocation of the Mig1 glucose repressor. Mol Biol Cell. 1997, 8: 1603-1618. 10.1091/mbc.8.8.1603.
Sun X, Zhang C, Dong J, Wu M, Zhang Y, Xiao D: Enhanced leavening properties of baker’s yeast overexpressingMAL62with deletion ofMIG1in lean dough. J Ind Microbiol Biotechnol. 2012, 39: 1533-1539. 10.1007/s10295-012-1144-7.
Klein CJ, Olsson L, Rønnow B, Mikkelsen JD, Nielsen J: Alleviation of glucose repression of maltose metabolism byMIG1disruption inSaccharomyces cerevisiae. Appl Environ Microbiol. 1996, 62: 4441-4449.
Treitel MA, Carlson M: Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc Natl Acad Sci U S A. 1995, 92: 3132-3136. 10.1073/pnas.92.8.3132.
Davie JK, Trumbly RJ, Dent SY: Histone-dependent association of Tup1-Ssn6 with repressed genes in vivo. Mol Cell Biol. 2002, 22: 693-703. 10.1128/MCB.22.3.693-703.2002.
Keleher CA, Redd MJ, Schultz J, Carlson M, Johnson AD: Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992, 68: 709-719. 10.1016/0092-8674(92)90146-4.
Hanlon SE, Rizzo JM, Tatomer DC, Lieb JD, Buck MJ: The stress response factors Yap6, Cin5, Phd1, and Skn7 direct targeting of the conserved co-repressor Tup1-Ssn6 in S. cerevisiae. PLoS One 2011, 6:e19060.
García–Sánchez S, Mavor AL, Russell CL, Argimon S, Dennison P, Enjalbert B, Brown AJ: Global roles of Ssn6 in Tup1- and Nrg1-dependent gene regulation in the fungal pathogen,Candida albicans. Mol Biol Cell. 2005, 16: 2913-2925. 10.1091/mbc.E05-01-0071.
Hu Z, Nehlin JO, Ronne H, Michels CA:MIG1-dependent andMIG1-independent glucose regulation ofMALgene expression inSaccharomyces cerevisiae. Curr Genet. 1995, 28: 258-266. 10.1007/BF00309785.
Klein CJL, Olsson L, Ronnow B, Mikkelsen JD, Nielsen J: Glucose and Maltose Metabolism inMIG1-disrupted andMAL-constitutive Strains ofSaccharomyces cerevisiae. Food Technol Biotechnol. 1997, 35: 287-292.
Schüller HJ: Transcriptional control of nonfermentative metabolism in the yeastSaccharomyces cerevisiae. Curr Genet. 2003, 43: 139-160.
Rolland F, Winderickx J, Thevelein JM: Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002, 2: 183-201. 10.1111/j.1567-1364.2002.tb00084.x.
Geladé R, Van de Velde S, Van Dijck P, Thevelein JM: Multi-level response of the yeast genome to glucose.Genome Biol2003, 4:233.
Olsson L, Nielsen J: The role of metabolic engineering in the improvement ofSaccharomyces cerevisiae: utilization of industrial media. Enzyme Microb Technol. 2000, 26: 785-792. 10.1016/S0141-0229(00)00172-1.
Klein CJ, Rasmussen JJ, Rønnow B, Olsson L, Nielsen J: Investigation of the impact ofMIG1andMIG2在the physiology ofSaccharomyces cerevisiae. J Biotechnol. 1999, 68: 197-212. 10.1016/S0168-1656(98)00205-3.
Williams FE, Varanasi U, Trumbly RJ: The CYC8 and TUP1 proteins involved in glucose repression inSaccharomyces cerevisiaeare associated in a protein complex. Mol Cell Biol. 1991, 11: 3307-3316.
Varanasi US, Klis M, Mikesell PB, Trumbly RJ: The Cyc8 (Ssn6)-Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits. Mol Cell Biol. 1996, 16: 6707-6714.
Carrico PM, Zitomer RS: Mutational analysis of the Tup1 general repressor of yeast. Genetics. 1998, 148: 637-644.
Tzamarias D, Struhl K: Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8-Tup1 corepressor complex to differentially regulated promoters. Genes Dev. 1995, 9: 821-831. 10.1101/gad.9.7.821.
Zhang Z, Varanasi U, Trumbly RJ: Functional dissection of the global repressor Tup1 in yeast: dominant role of the C-terminal repression domain. Genetics. 2002, 161: 957-969.
Lamas–Maceiras M, Freire–Picos MA, Torres AM: Transcriptional repression byKluyveromyces lactisTup1 inSaccharomyces cerevisiae. J Ind Microbiol Biotechnol. 2011, 38: 79-84. 10.1007/s10295-010-0832-4.
Lee KS, Hong ME, Jung SC, Ha SJ, Yu BJ, Koo HM, Park SM, Seo JH, Kweon DH, Park JC, Jin YS: Improved galactose fermentation ofSaccharomyces cerevisiaethrough inverse metabolic engineering. Biotechnol Bioeng. 2011, 108: 621-631. 10.1002/bit.22988.
Wong KH, Struhl K: The Cyc8-Tup1 complex inhibits transcription primarily by masking the activation domain of the recruiting protein. Genes Dev. 2011, 25: 2525-2539. 10.1101/gad.179275.111.
Papamichos-Chronakis M, Gligoris T, Tzamarias D: The Snf1 kinase controls glucose repression in yeast by modulating interactions between the Mig1 repressor and the Cyc8-Tup1 co-repressor. EMBO Rep. 2004, 5: 368-372. 10.1038/sj.embor.7400120.
Vallier LG, Carlson M: Synergistic release from glucose repression bymig1andssnmutations inSaccharomyces cerevisiae. Genetics. 1994, 137: 49-54.
Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH: A second set ofloxPmarker cassettes forCre-mediated multiple gene knockouts in budding yeast.Nucleic Acids Res2002, 30:e23.
Lu J, Dong J, Wu D, Chen Y, Guo X, Shi Y, Sun X, Xiao D: Construction of recombinant industrial brewer’s yeast with lower diacetyl production and proteinase A activity. Eur Food Res Technol. 2012, 235: 951-961. 10.1007/s00217-012-1821-9.
Gietz RD, Woods RA: Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002, 350: 87-96. 10.1016/S0076-6879(02)50957-5.
Hauf J, Zimmermann FK, Müller S: Simultaneous genomic overexpression of seven glycolytic enzymes in the yeastSaccharomyces cerevisiae. Enzyme Microb Technol. 2000, 26: 688-698. 10.1016/S0141-0229(00)00160-5.
Acknowledgments
The current study was financially supported by the National High Technology Research and Development Program of China (2013AA102106), the National Natural Science Foundation of China (31171730; 31000043), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1166).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XL carried out the experiments and drafted the manuscript. XWB and HYS participated in the plasmid and strain construction. CYZ and DGX conceived the study and reviewed the final manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visithttps://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, X., Zhang, CY., Bai, XW.et al.Effects ofMIG1,TUP1andSSN6deletion on maltose metabolism and leavening ability of baker’s yeast in lean dough.Microb Cell Fact13, 93 (2014). https://doi.org/10.1186/s12934-014-0093-4
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s12934-014-0093-4