- Research
- Open access
- Published:
Microalgae as bioreactors for bioplastic production
188bet亚洲体育平台volume10, Article number:81(2011)
Abstract
Background
Poly-3-hydroxybutyrate (PHB) is a polyester with thermoplastic properties that is naturally occurring and produced by such bacteria asRalstonia eutrophaH16 andBacillus megaterium. In contrast to currently utilized plastics and most synthetic polymers, PHB is biodegradable, and its production is not dependent on fossil resources making this bioplastic interesting for various industrial applications.
Results
In this study, we report on introducing the bacterial PHB pathway ofR. eutrophaH16 into the diatomPhaeodactylum tricornutum, thereby demonstrating for the first time that PHB production is feasible in a microalgal system. Expression of the bacterial enzymes was sufficient to result in PHB levels of up to 10.6% of algal dry weight. The bioplastic accumulated in granule-like structures in the cytosol of the cells, as shown by light and electron microscopy.
Conclusions
Our studies demonstrate the great potential of microalgae like the diatomP. tricornutumto serve as solar-powered expression factories and reveal great advantages compared to plant based production systems.
Background
About 140 million tons of plastic are consumed every year worldwide, which necessitates the processing of approximately 150 million tons of fossil fuels and directly causes immense amounts of waste that can take thousands of years to naturally deteriorate, if it degrades at all [118bet金博宝app ].Consequently, bioplastics are a feasible alternative in that they are not based on fossil resources and can easily be biodegraded. So far, however, production costs for petroleum-derived polymers still remain lower than biodegradable alternatives, which is a hindrance to commercial development and retail of environmentally friendly alternatives.
Poly-(R)-3-hydroxybutyrate (PHB) is an aliphatic polyester with thermoplastic properties, which is naturally produced by certain bacteria as storage compound and is 100% biodegradable [118bet金博宝app –118bet金博宝app ].PHB is synthesized from acetyl-CoA by the action of three enzymes: a ketothiolase, an acetoacetyl-CoA reductase and a PHB synthase [118bet金博宝app ].Under optimal conditions bacteria such asRalstonia eutrophaH16 can produce up to 80% PHB of cellular dry weight, and some companies have specialized on commercial PHB production (e.g. Metabolix Inc., Micromidas Inc.). Nevertheless, costs for PHB production by bacterial fermentation are still very high, which brought plants into focus as photosynthesis fueled low-cost production system [118bet金博宝app –118bet金博宝app ].The three bacterial enzymes were expressed in the cytosol or targeted to different compartments of the plant cell leading to high amounts of PHB accumulation in the plastids ofArabidopsis thaliana(up to 40% of dry weight) [118bet金博宝app ,118bet金博宝app ].However, due to stunted growth and infertility, these plants were not suitable for large-scale cultivation. Today, the highest levels of PHB synthesis in plants with fertile offspring are obtained in the plastid ofNicotiana tabacumresulting in up to 18% PHB of cellular dry weight [118bet金博宝app ].
In general, plant-based expression systems are very attractive in that no external organic carbon source is required, which is quite an important cost factor for large-scale production systems [118bet金博宝app –118bet金博宝app ].然而,另一方面,植物性表达ion systems compete directly with subsistence crops for agricultural acreage and the dissemination of transgenic plants is difficult to control, which has been an ethical concern and led to strict regulatory controls of transgenic plants in many countries. Major drawbacks for the establishment of plant-based expression systems are the comparatively long growth rates resulting in a decrease in profitability. This casts a shadow on plant-based expression systems, rendering them unable to compete with the current well-established bacterial systems. Microalgae share all the advantages of photosynthetically driven eukaryotic systems but lack many of the mentioned disadvantages i.e. they possess high growth rates, are easy to handle and do not need much more than light and water for cultivation [118bet金博宝app ].Thus, microalgae are thought to have great potential as novel low-cost expression systems especially if aiming at the biosynthesis of recombinant proteins needed in numerous industrial, therapeutic or diagnostic applications [118bet金博宝app –118bet金博宝app ].
In this study, we present for the first time a report on the biosynthesis of a biotechnologically relevant biopolymer in a microalgal system. We demonstrate that production of the bioplastic PHB is feasible in the diatomPhaeodactylum tricornutumby introducing the bacterial PHB pathway into the cytosolic compartment. PHB levels of up to 10.6% of algal dry weight were obtained revealing the great potential of this low-cost and environmentally friendly expression system.
Results and Discussion
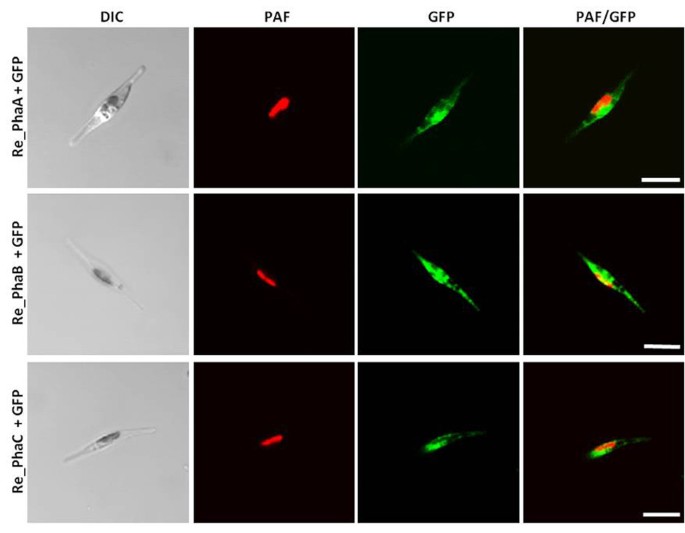
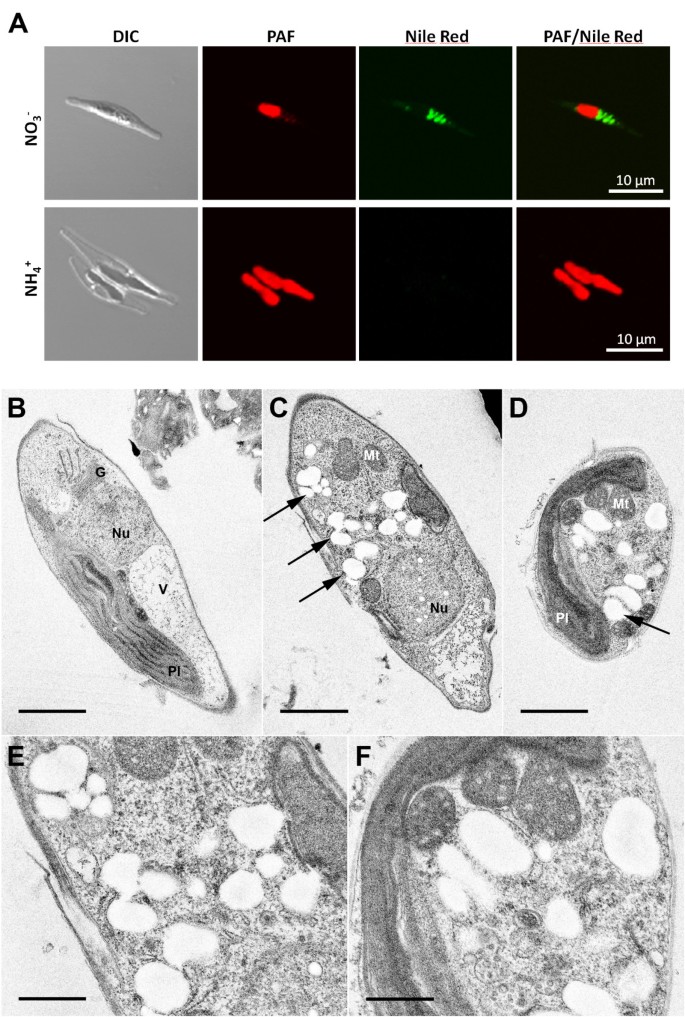
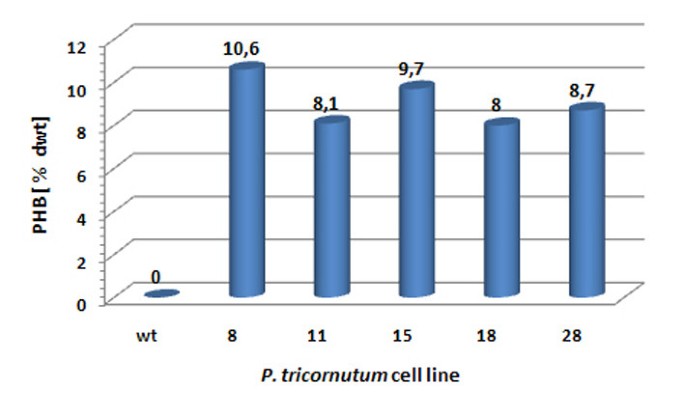
The enzymes PhaA (ketothiolase), PhaB (acetoacetyl-CoA reductase) and PhaC (PHB synthase) of the Gram-negative bacteriumR. eutrophaH16 were expressed in the cytosol of the diatomP. tricornutumto test whether polyhydroxybutyrate (PHB) can be produced in a microalgal system. Initialin vivolocalization studies with GFP fusion proteins demonstrated that all three enzymes are expressed in the heterologous system and accumulate within the cytosol (Figure118bet金博宝app ).P. tricornutumcells that were co-transfected with sequences for all three enzymes being under the control of a nitrate-inducible promoter were first analyzed via PCR for the integration of all three constructs and were subsequently checked for morphological anomalies. Interestingly, when transferred to nitrate containing medium for 5-7 days (inducing the expression of PhaA, PhaB and PhaC) transfectants accumulated large amounts of granule-like structures within the cytosol, which were specifically labeled with the lipophilic dye Nile Red used in many other studies forin vivostaining of PHB granules (Figure118bet金博宝app ). Electron microscopic analyses confirmed these data demonstrating that cells were filled with granules that are not present in wild type cells or non-induced cells of the same line (Figure118bet金博宝app ). Gas chromatographic analyses ofP. tricornutum phaA/phaB/phaC-transfectants transferred to nitrate-containing medium for 7 days revealed that such cells indeed accumulate PHB to levels of up to 10.6% of algal dry weight (Figure118bet金博宝app ). Thereby, PHB accumulation turned out to be dependent on the induction period as only 1-3 days of PhaA/PhaB/PhaC expression resulted in much lower PHB quantities (data not shown). Importantly, wild type cells did not accumulate PHB by natural means (Figure118bet金博宝app ).
In vivolocalization studies on PhaA, PhaB and PhaC expression inP. tricornutum. Sequences for PhaA (ketothiolase), PhaB (acetoacetyl-CoA reductase) and PhaC (PHB synthase) ofR. eutrophaH16 were introduced as GFP fusion proteins inP. tricornutum. All three enzymes were expressed in the heterologous system and accumulate in the cytosol as no specific targeting signal was added. Plastid autofluorescence is shown in red and GFP fluorescence is depicted in green. Scale bar represents 10 μm. PAF - plastid autofluorescence, Re -R. eutrophaH16
Fluorescence and electron microscopic analyses on PHB accumulation inP. tricornutum. Cytosolic expression of enzymes PhaA, PhaB and PhaC ofR. eutrophaH16 induces the formation of granule-like structures that are stained by the lipophilic dye Nile red as visualized by fluorescence microscopy (A: NO3-). Under non-induced conditions no such granules were observed (A: NH4+). Electron microscopic analyses confirm cytosolic accumulation of electron-translucent granules (exemplarily marked by arrows) in cell lines expressing bacterial enzymes of the PHB pathway (C-F). PHB granules are about 0.1-0.3 μm in size and were not observed under non-induced conditions (B). Scale bar represents 1 μm (B-D) and 500 nm (E/F). DIC - differential interference contrast, G - golgi apparatus, Mt -mitochondrium, Nu - nucleus, PAF - plastid autofluorescence, Pl - plastid, V - vacuole
Quantification of PHB synthesis in the cytosol ofP. tricornutum. The level of PHB synthesis for five transgenicP. tricornutumcell lines (No. 8, 11, 15, 18, 28) was analyzed by gas chromatography coupled to mass spectrometry. After 7 days of PhaA/PhaB/PhaC expression PHB levels of 8.0 to 10.6% of algal dry weight were detected. Wild type cells were negative for PHB synthesis. dwt - dry weight, wt - wild type
The results of this study show for the very first time that PHB production is possible in a microalgae system. Interestingly, in comparison to efforts on PHB synthesis in the cytosol of plants, PHB expression levels inP. tricornutumare about 100-fold higher [118bet金博宝app ].This might be due to large lipid deposits present in the cytosol ofP. tricornutum, as these microalgae naturally produce valuable omega-3-fatty acids [118bet金博宝app –118bet金博宝app ].Therefore, the acetyl-CoA pool, which is the basis for PHB synthesis, might be notably high in the cytosol ofP. tricornutumand hence enable very efficient PHB production. To circumvent acetyl-CoA limitations as a drawback for PHB production in plants, other cellular compartments were tested, and indeed plastids, which provide a high acetyl-CoA content because of fatty acid synthesis, turned out to produce much higher levels of PHB [118bet金博宝app ,118bet金博宝app ].The best PHB synthesis levels in plants with fertile offspring thus far were achieved in tobacco with PHB contributing to 18% of dry weight [118bet金博宝app ].Upon a first glance, this looks promising, however, taking production time as an important economic factor into account plants do poorly in direct comparison toP. tricornutum, which needs approximately two weeks to accumulate similar PHB levels reached by plants during a vegetation period of 3 months.
Of course PHB production inP. tricornutumcannot presently compete with bioplastic production inR. eutropha, which was established commercially many years ago. Nevertheless, this pilot experiment together with many other current projects on microalgal biotechnology highlights the immense potential of these photosynthetically driven production systems. Surely, such progress in microalgal biotechnology will boost the development of efficient photobioreactors for use in large-scale cultivation, which is currently one of the most limiting factors to put low-cost production into practice.
Conclusions
Altogether, this study has demonstrated that microalgae like the diatomP. tricornutumhave a great potential not only as biosynthetic factory for recombinant proteins but also as photosynthetically fueled bioreactors for synthesizing biotechnologically relevant polymers like PHB. Even though no enzyme engineering, no adaptations toP. tricornutumspecific codon-usage, and no large-scale screening have been applied in these initial analyses, relatively high PHB levels of up to 10.6% of algal dry weight have been obtained. Thus, in the future, there will be a focus on various targets for enhancing PHB biosynthesis inP. tricornutum. Other subcellular compartments such as the plastids might yet be interesting sites for PHB synthesis. Diatoms are naturally rich in lipids and silicate and already have applications in biotechnology [118bet金博宝app ].Hence, inserting and/or altering biochemical pathways in diatoms in order to synthesize complex molecules, biologically active substances, and raw materials may have a number of applications in for example the nanotechnology industry and the production of renewable biofuels.
Methods
Plasmid construction andP. tricornutumtransfection
Plasmid pBHR68 [118bet金博宝app ] was used as template for amplification ofphaA,phaBandphaCgenes fromR. eutrophaH16. Forin vivolocalization studies sequences were cloned upstream to the eGFP (enhanced green fluorescent protein) sequence into the vector pPha-NR, which is a derivative of pPhaT1 with endogenous nitrate reductase promoter/terminator flanking the multiple cloning site [GenBank:JN180663]. The inducible nitrate reductase promoter system was established earlier in the diatomC. fusiformisby Poulsen et al. 2005 [118bet金博宝app ].Transfection proceeded as described previously [118bet金博宝app ] with the exception that cells were grown under non-induced conditions with NH4+as sole nitrogen source. For PHB synthesis inP. tricornutum的序列phaCwas cloned into the vector pPha-NR (not containing eGFP), and sequences ofphaAandphaBwere inserted into the vector pPha-DUAL[2xNR], which is a pPha-NR derivative with two multiple cloning sites both under the control of endogenous nitrate reductase promoter [GenBank:JN180664]. Both plasmids were mixed and co-transfected under non-induced conditions.
Cell culture and induction of recombinant protein expression
Cells were grown in f/2 medium under standard conditions as described elsewhere (Apt et al. 1999) with either 0.9 mM NO3-or 1.5 mM NH4+as the nitrogen source. Forin vivolocalization studies on GFP fusion proteins transfectants were grown in media containing NO3-to induce recombinant protein expression. After 3 days, clones were analyzed by confocal laser scanning microscopy.PhaA/phaB/phaCco-transfectants were first determined to have genomic integration by colony PCR for all three sequences. Subsequently, positive colonies were grown in liquid culture containing NH4+and allowed to reach exponential phase, whereupon they were transferred to NO3-containing medium for varying time periods. For visualization of PHB granules, cells were induced for 5 days and analyzed by electron and confocal microscopic analyses, respectively. For confocal microscopy, cells were pre-incubated with the lipophilic dye Nile Red (0.5 μg/ml) for 24 h.
PHB analyses
For analyses on PHB production cultures were grown in NH4+containing medium, washed in nitrogen-free medium and transferred to NO3-containing medium for 7 days. Cells were harvested (1500 ×g, 10 min), washed with phosphate buffered saline (PBS) and lyophilized for 24 hours. The PHB contents of the cells were determined upon methanolysis of 5 to 10 mg lyophilized cells in presence of 2 ml methanol/sulfuric acid (85:15, v/v) and 2 ml chloroform. The resulting methyl esters of 3-hydroxybutyrate were analysed by gas chromatography using an Agilent 6850 GC (Agilent Technologies, Waldbronn, Germany) as described previously [118bet金博宝app ,118bet金博宝app ].
Fluorescence and electron microscopy
In vivo本地化的GFP融合蛋白进行了分析ith a confocal laser scanning microscope Leica TCS SP2 using a HCX PLAPO 63x/1.32-0.6 oil Ph3 CS objective. GFP, chlorophyll and Nile Red were excited at 488 nm, and fluorescence was detected at a bandwidth of 500-520 nm, 680-720 nm and 580-600 nm, respectively. For electron microscopic analyses, cells were centrifuged at 2000 × g for 5 min followed by cryo-fixation and resin embedding. The samples were high-pressure frozen in a Leica EM-PACT 2 and subsequently freeze substituted (Leica EM AFS 2; Leica, Vienna, Austria) with pure acetone containing 2% (w/v) osmium tetroxide, 0.1% (w/v) uranyl acetate and 5% (v/v) H2O. Freeze substitution was carried out at -90°C for 4 h, -60°C for 8 h, -30°C for 8 h and held at 0°C for 3 h with a heating time of 1 h in between each step. After washing the samples with ice cold acetone for three times and infiltration in Epon 812 (Ted Pella, Inc., USA) for 24 h, the resin was polymerized at 60°C for 72 h. Ultrathin sections were cut with a Leica Ultracut (Leica, Vienna, Austria), mounted on uncoated 400 mesh copper grids and post-stained with 2% (w/v) uranyl acetate for 20 min and 0.5% (w/v) lead citrate for 1 min. Transmission electron microscopy was carried out on a JEOL 2100 TEM operated at 80 kV in combination with a fast-scan 2 k × 2 k CCD camera F214 (TVIPS, Gauting, Germany).
References
Suriyamongkol P, Weselake R, Narine S, Moloney M, Shah S: Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants - a review. Biotechnol Adv. 2007, 25 (2): 148-175. 10.1016/j.biotechadv.2006.11.007.
Poirier Y, Nawrath C, Somerville C: Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Biotechnology (N Y). 1995, 13 (2): 142-150. 10.1038/nbt0295-142.
Steinbüchel A, Füchtenbusch B: Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998, 16 (10): 419-427. 10.1016/S0167-7799(98)01194-9.
Steinbüchel A, Hein S: Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv Biochem Eng Biotechnol. 2001, 71: 81-123.
Madison LL, Huisman GW: Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999, 63 (1): 21-53.
Steinbüchel A, Schlegel HG: Physiology and molecular genetics of poly(beta-hydroxy-alkanoic acid) synthesis inAlcaligenes eutrophus. Mol Microbiol. 1991, 5 (3): 535-542. 10.1111/j.1365-2958.1991.tb00725.x.
Poirier Y, Dennis DE, Klomparens K, Somerville C: Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science. 1992, 256 (5056): 520-523. 10.1126/science.256.5056.520.
Mittendorf V, Robertson EJ, Leech RM, Kruger N, Steinbüchel A, Poirier Y: Synthesis of medium-chain-length polyhydroxyalkanoates inarabidopsis thalianausing intermediates of peroxisomal fatty acid beta-oxidation. Proc Natl Acad Sci USA. 1998, 95 (23): 13397-13402. 10.1073/pnas.95.23.13397.
Snell KD, Peoples OP: Polyhydroxyalkanoate polymers and their production in transgenic plants. Metab Eng. 2002, 4 (1): 29-40. 10.1006/mben.2001.0214.
van Beilen JB, Poirier Y: Production of renewable polymers from crop plants. Plant J. 2008, 54 (4): 684-701. 10.1111/j.1365-313X.2008.03431.x.
Bohmert K, Balbo I, Kopka J, Mittendorf V, Nawrath C, Poirier Y, Tischendorf G, Trethewey RN, Willmitzer L: Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4% of their fresh weight. Planta. 2000, 211 (6): 841-845. 10.1007/s004250000350.
Nawrath C, Poirier Y, Somerville C: Targeting of the polyhydroxybutyrate biosynthetic pathway to the plastids ofArabidopsis thalianaresults in high levels of polymer accumulation. Proc Natl Acad Sci USA. 1994, 91 (26): 12760-12764. 10.1073/pnas.91.26.12760.
Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD: High levels of bioplastic are produced in fertile transplastomic tobacco plants engineered with a synthetic operon for the production of polyhydroxybutyrate. Plant Physiol. 2011, 155 (4): 1690-1708. 10.1104/pp.110.169581.
Daniell H, Singh ND, Mason H, Streatfield SJ: Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009, 14 (12): 669-679. 10.1016/j.tplants.2009.09.009.
Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM: Plant-based production of biopharmaceuticals. Curr Opin Plant Biol. 2004, 7 (2): 152-158. 10.1016/j.pbi.2004.01.007.
Goddijn OJM, Pen J: Plants as bioreactors. Trends in Biotechnology. 1995, 13 (9): 379-387. 10.1016/S0167-7799(00)88985-4.
Bozarth A, Maier UG, Zauner S: Diatoms in biotechnology: modern tools and applications. Appl Microbiol Biotechnol. 2009, 82 (2): 195-201. 10.1007/s00253-008-1804-8.
Mayfield SP, Manuell AL, Chen S, Wu J, Tran M, Siefker D, Muto M, Marin-Navarro J:Chlamydomonas reinhardtiichloroplasts as protein factories. Curr Opin Biotechnol. 2007, 18 (2): 126-133. 10.1016/j.copbio.2007.02.001.
Potvin G, Zhang Z: Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv. 2010, 28 (6): 910-918. 10.1016/j.biotechadv.2010.08.006.
Rasala BA, Muto M, Lee PA, Jager M, Cardoso RM, Behnke CA, Kirk P, Hokanson CA, Crea R, Mendez M, et al: Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast ofChlamydomonas reinhardtii. Plant Biotechnol J. 2010, 8 (6): 719-733. 10.1111/j.1467-7652.2010.00503.x.
Walker TL, Purton S, Becker DK, Collet C: Microalgae as bioreactors. Plant Cell Rep. 2005, 24 (11): 629-641. 10.1007/s00299-005-0004-6.
Carsten M, Molina Grima E, Robles Medina A, Giménez Giménez A, Ibánez González MJ: Eicosapentaenoic acid (EPA, 20:5n3) from the microalgaPhaeodactylum tricornutum. J Am Oil chem Soc. 1996, 73: 1025-1031. 10.1007/BF02523411.
Ramírez Fajardo A, Esteban Cerdán L, Robles Medina A, Acién Fernández FG, González Moreno PA, Molina Grima E: Lipid extraction from the microalgaPhaeodactylum tricornutum. Eur J Lipid Sci Technol. 2007, 109: 120-126. 10.1002/ejlt.200600216.
Yongmanitchai W, Ward OP: Growth of and omega-3 fatty acid production byPhaeodactylum tricornutumunder different culture conditions. Appl Environ Microbiol. 1991, 57 (2): 419-425.
Spiekermann P, Rehm BH, Kalscheuer R, Baumeister D, Steinbüchel A: A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol. 1999, 171 (2): 73-80. 10.1007/s002030050681.
Poulsen N, Kroger N: A new molecular tool for transgenic diatoms: control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J. 2005, 272 (13): 3413-3423. 10.1111/j.1742-4658.2005.04760.x.
Apt KE, Kroth-Pancic PG, Grossman AR: Stable nuclear transformation of the diatomPhaeodactylum tricornutum. Mol Gen Genet. 1996, 252 (5): 572-579.
Brandl H, Gross RA, Lenz RW, Fuller RC:Pseudomonas oleovoransas a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988, 54 (8): 1977-1982.
Timm A, Steinbüchel A: Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate byPseudomonas aeruginosaand other fluorescent pseudomonads. Appl Environ Microbiol. 1990, 56 (11): 3360-3367.
Acknowledgements
We are grateful to Jan Bamberger from Marburg for his technical assistance in initial gas chromatographic analyses on PHB accumulation. Prof. Reinhard Rachel and Prof. Ralph Witzgall from Regensburg we thank for providing high pressure freezing and freeze substitution facilities. Furthermore, we thank Marion Debus for technical assistance with electron microscopic preparations. This work was supported by the LOEWE program of the state of Hessen (Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
研究设计了UGM, ASB和跳频。问conducted experimental work on PHB-analyses and AK performed electron microscopic analyses. FH is responsible for design and drafting of the manuscript and carried out further experimental work. AS, UL and SZ assisted in data analysis and review of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access这篇文章发表在license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hempel, F., Bozarth, A.S., Lindenkamp, N.et al.Microalgae as bioreactors for bioplastic production.Microb Cell Fact10, 81 (2011). https://doi.org/10.1186/1475-2859-10-81
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/1475-2859-10-81
Comments
View archived comments (1)